Use the Mo Diagram Provided Below to Answer the Following Questions: What Is the Bond Order for C2?
Chapter viii. Advanced Theories of Covalent Bonding
8.4 Molecular Orbital Theory
Learning Objectives
Past the end of this section, you volition be able to:
- Outline the basic quantum-mechanical approach to deriving molecular orbitals from atomic orbitals
- Describe traits of bonding and antibonding molecular orbitals
- Calculate bond orders based on molecular electron configurations
- Write molecular electron configurations for first- and second-row diatomic molecules
- Relate these electron configurations to the molecules' stabilities and magnetic properties
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. All the same, one of the almost important molecules we know, the oxygen molecule O2, presents a trouble with respect to its Lewis structure. We would write the following Lewis construction for O2:
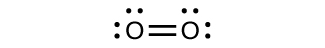
This electronic structure adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen cantlet has viii electrons around it. However, this flick is at odds with the magnetic behavior of oxygen. Past itself, Otwo is non magnetic, simply it is attracted to magnetic fields. Thus, when we cascade liquid oxygen by a stiff magnet, it collects between the poles of the magnet and defies gravity, as in Figure one in Chapter 8 Introduction. Such attraction to a magnetic field is called paramagnetism, and it arises in molecules that accept unpaired electrons. And still, the Lewis structure of O2 indicates that all electrons are paired. How do we account for this discrepancy?
Magnetic susceptibility measures the force experienced past a substance in a magnetic field. When nosotros compare the weight of a sample to the weight measured in a magnetic field (Figure i), paramagnetic samples that are attracted to the magnet will appear heavier because of the force exerted by the magnetic field. We can calculate the number of unpaired electrons based on the increase in weight.
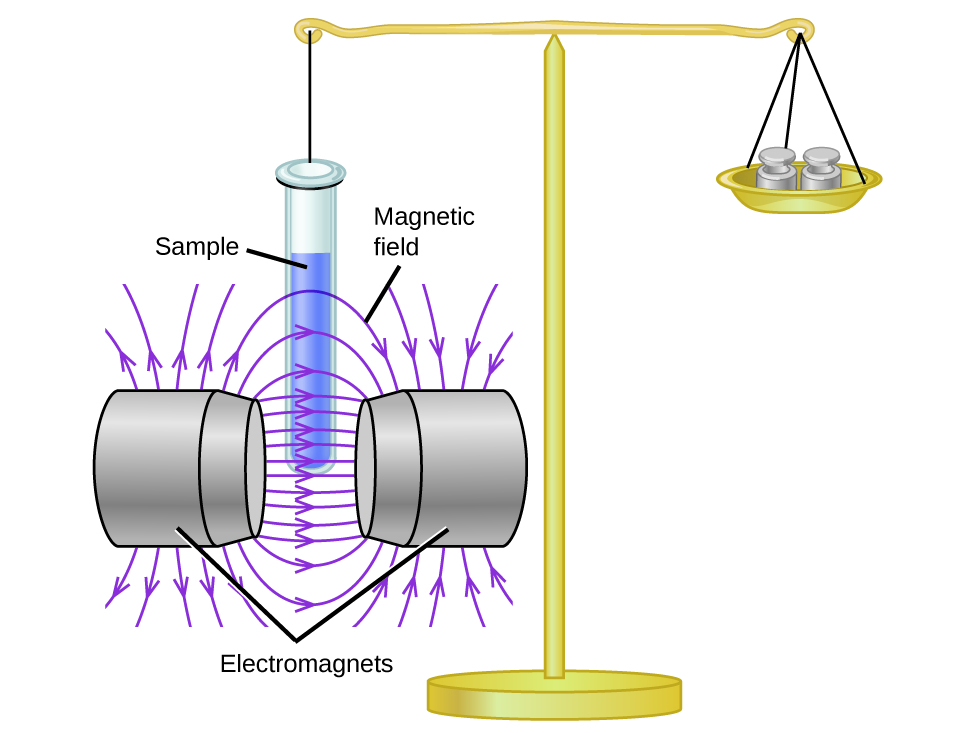
Experiments evidence that each O2 molecule has two unpaired electrons. The Lewis-structure model does not predict the presence of these two unpaired electrons. Different oxygen, the apparent weight of near molecules decreases slightly in the presence of an inhomogeneous magnetic field. Materials in which all of the electrons are paired are diamagnetic and weakly repel a magnetic field. Paramagnetic and diamagnetic materials do not human activity as permanent magnets. Only in the presence of an applied magnetic field do they demonstrate attraction or repulsion.

Water, like most molecules, contains all paired electrons. Living things contain a large percentage of h2o, so they demonstrate diamagnetic behavior. If you identify a frog near a sufficiently large magnet, it will levitate. You tin can see videos of diamagnetic floating frogs, strawberries, and more.
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures. Additionally, information technology provides a model for describing the energies of electrons in a molecule and the likely location of these electrons. Unlike valence bail theory, which uses hybrid orbitals that are assigned to one specific atom, MO theory uses the combination of atomic orbitals to yield molecular orbitals that are delocalized over the entire molecule rather than being localized on its constituent atoms. MO theory likewise helps us understand why some substances are electrical conductors, others are semiconductors, and however others are insulators. Tabular array 2 summarizes the main points of the 2 complementary bonding theories. Both theories provide different, useful ways of describing molecular structure.
| Valence Bond Theory | Molecular Orbital Theory |
|---|---|
| considers bonds as localized between one pair of atoms | considers electrons delocalized throughout the entire molecule |
| creates bonds from overlap of atomic orbitals (south, p, d…) and hybrid orbitals (sp, sp 2, sp 3…) | combines atomic orbitals to course molecular orbitals (σ, σ*, π, π*) |
| forms σ or π bonds | creates bonding and antibonding interactions based on which orbitals are filled |
| predicts molecular shape based on the number of regions of electron density | predicts the arrangement of electrons in molecules |
| needs multiple structures to draw resonance | |
| Table ii. Comparison of Bonding Theories | |
Molecular orbital theory describes the distribution of electrons in molecules in much the same manner that the distribution of electrons in atoms is described using diminutive orbitals. Using quantum mechanics, the behavior of an electron in a molecule is all the same described past a wave function, Ψ, analogous to the beliefs in an atom. Just like electrons around isolated atoms, electrons around atoms in molecules are limited to discrete (quantized) energies. The region of space in which a valence electron in a molecule is likely to be found is chosen a molecular orbital (Ψ two). Like an atomic orbital, a molecular orbital is full when it contains two electrons with contrary spin.
Nosotros will consider the molecular orbitals in molecules equanimous of two identical atoms (H2 or Cl2, for example). Such molecules are called homonuclear diatomic molecules. In these diatomic molecules, several types of molecular orbitals occur.
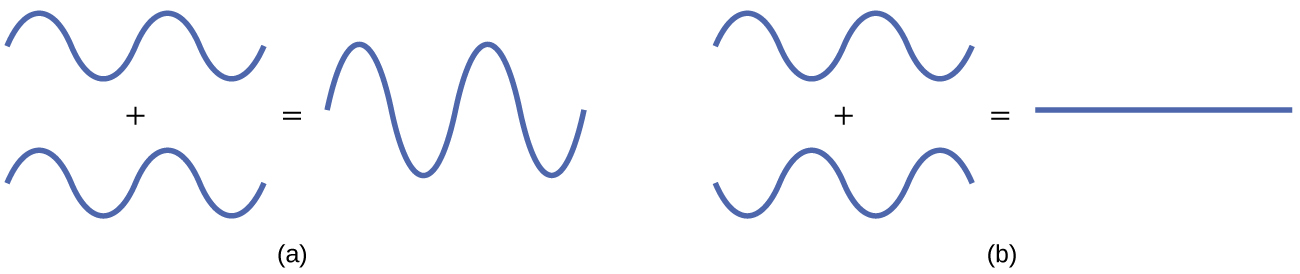
The mathematical process of combining atomic orbitals to generate molecular orbitals is called the linear combination of diminutive orbitals (LCAO). The wave function describes the wavelike properties of an electron. Molecular orbitals are combinations of atomic orbital wave functions. Combining waves tin can atomic number 82 to effective interference, in which peaks line upwards with peaks, or subversive interference, in which peaks line upward with troughs (Figure 2). In orbitals, the waves are iii dimensional, and they combine with in-stage waves producing regions with a higher probability of electron density and out-of-phase waves producing nodes, or regions of no electron density.
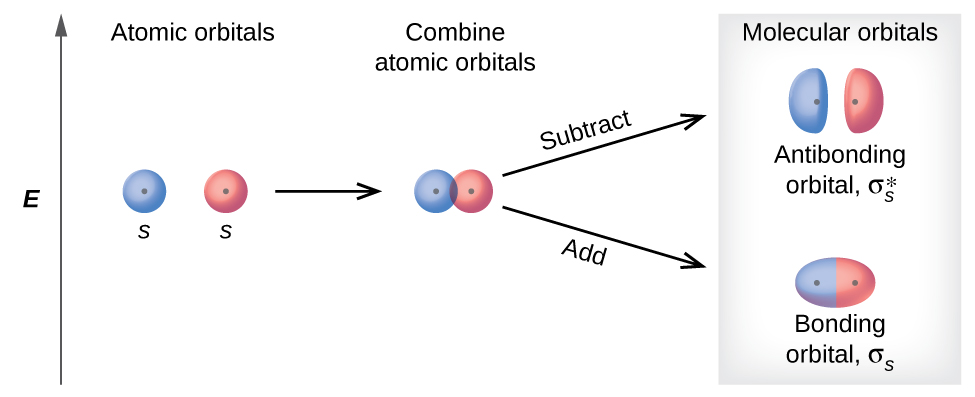
There are two types of molecular orbitals that can form from the overlap of ii atomic southward orbitals on adjacent atoms. The two types are illustrated in Figure 3. The in-phase combination produces a lower free energy σ due south molecular orbital (read as "sigma-due south") in which most of the electron density is directly between the nuclei. The out-of-phase add-on (which can likewise exist thought of as subtracting the wave functions) produces a college energy [latex]\pmb \sigma^*_s[/latex]molecular orbital (read every bit "sigma-s-star") molecular orbital in which there is a node between the nuclei. The asterisk signifies that the orbital is an antibonding orbital. Electrons in a σ s orbital are attracted by both nuclei at the aforementioned time and are more stable (of lower energy) than they would be in the isolated atoms. Calculation electrons to these orbitals creates a forcefulness that holds the two nuclei together, so we call these orbitals bonding orbitals. Electrons in the [latex]\sigma^*_s[/latex] orbitals are located well away from the region between the two nuclei. The attractive force between the nuclei and these electrons pulls the two nuclei apart. Hence, these orbitals are called antibonding orbitals. Electrons fill the lower-energy bonding orbital before the higher-free energy antibonding orbital, just as they fill lower-energy diminutive orbitals before they fill up higher-energy atomic orbitals.

Y'all can scout animations visualizing the calculated atomic orbitals combining to form diverse molecular orbitals at the Orbitron website.
In p orbitals, the wave function gives ascent to two lobes with opposite phases, analogous to how a 2-dimensional wave has both parts above and below the average. Nosotros indicate the phases past shading the orbital lobes unlike colors. When orbital lobes of the same phase overlap, constructive wave interference increases the electron density. When regions of opposite phase overlap, the destructive moving ridge interference decreases electron density and creates nodes. When p orbitals overlap cease to finish, they create σ and σ* orbitals (Figure 4). If 2 atoms are located along the x-axis in a Cartesian coordinate system, the two px orbitals overlap end to end and form σ px (bonding) and [latex]\sigma^*_{px}[/latex] (antibonding) (read as "sigma-p-x" and "sigma-p-x star," respectively). Only as with due south-orbital overlap, the asterisk indicates the orbital with a node betwixt the nuclei, which is a higher-energy, antibonding orbital.
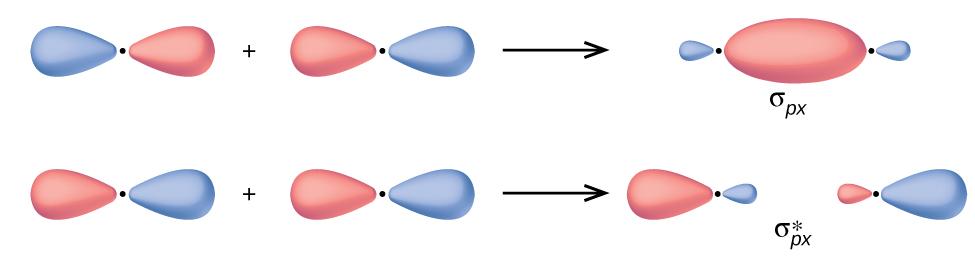
The side-by-side overlap of 2 p orbitals gives rising to a pi (π) bonding molecular orbital and a π* antibonding molecular orbital, equally shown in Effigy 5. In valence bond theory, we depict π bonds every bit containing a nodal plane containing the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. In molecular orbital theory, we describe the π orbital by this same shape, and a π bond exists when this orbital contains electrons. Electrons in this orbital interact with both nuclei and assistance hold the two atoms together, making it a bonding orbital. For the out-of-phase combination, at that place are ii nodal planes created, one along the internuclear centrality and a perpendicular one between the nuclei.
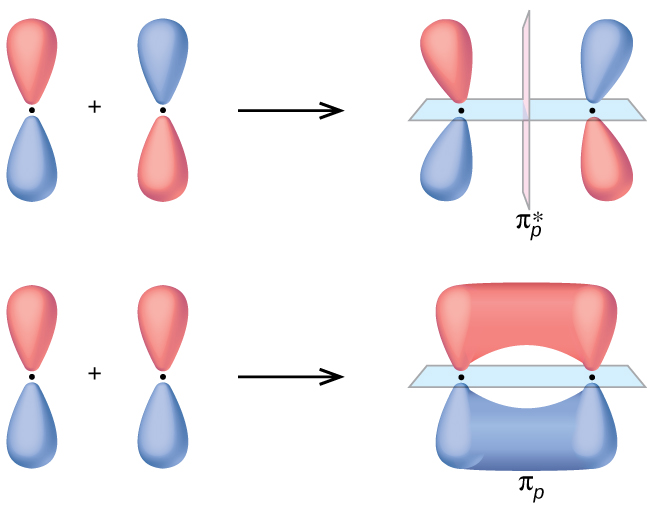
In the molecular orbitals of diatomic molecules, each atom besides has two sets of p orbitals oriented side past side (py and pz ), so these four diminutive orbitals combine pairwise to create two π orbitals and two π* orbitals. The π py and [latex]\pi^*_{py}[/latex] orbitals are oriented at correct angles to the π pz and [latex]\pi^*_{pz}[/latex] orbitals. Except for their orientation, the π py and π pz orbitals are identical and accept the same energy; they are degenerate orbitals. The [latex]\pi^*_{py}[/latex] and [latex]\pi^*_{px}[/latex] antibonding orbitals are also degenerate and identical except for their orientation. A full of six molecular orbitals results from the combination of the six atomic p orbitals in ii atoms: σ px and [latex]\sigma^*_{px}[/latex], π py and [latex]\pi^*_{py}[/latex], π pz and [latex]\pi^*_{pz}[/latex].
Instance 1
Molecular Orbitals
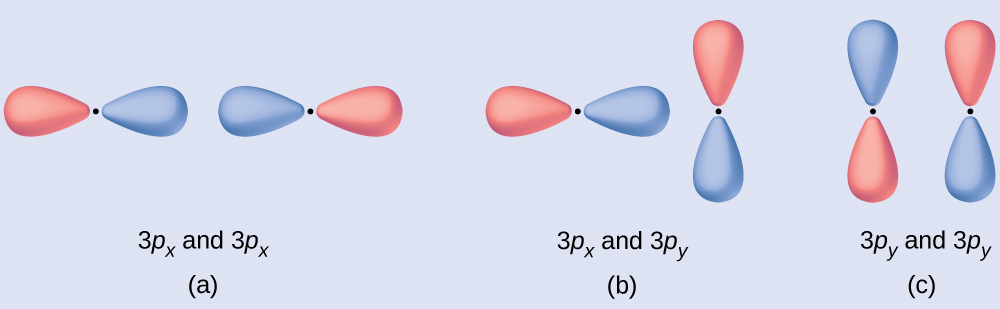
Predict what type (if any) of molecular orbital would issue from adding the wave functions so each pair of orbitals shown overlap. The orbitals are all similar in energy.
Solution
(a) is an in-phase combination, resulting in a σ3p orbital
(b) volition not upshot in a new orbital considering the in-phase component (bottom) and out-of-phase component (top) cancel out. Only orbitals with the correct alignment can combine.
(c) is an out-of-phase combination, resulting in a [latex]\pi^*_{3p}[/latex] orbital.
Check Your Learning
Label the molecular orbital shown every bit σ or π, bonding or antibonding and indicate where the node occurs.

Answer:
The orbital is located forth the internuclear axis, so it is a σ orbital. There is a node bisecting the internuclear axis, then it is an antibonding orbital.

Walter Kohn: Nobel Laureate
Walter Kohn (Figure 6) is a theoretical physicist who studies the electronic structure of solids. His work combines the principles of quantum mechanics with advanced mathematical techniques. This technique, called density functional theory, makes it possible to compute properties of molecular orbitals, including their shape and energies. Kohn and mathematician John Pople were awarded the Nobel Prize in Chemistry in 1998 for their contributions to our understanding of electronic structure. Kohn likewise made significant contributions to the physics of semiconductors.

Kohn's biography has been remarkable exterior the realm of physical chemical science every bit well. He was built-in in Republic of austria, and during Globe War II he was part of the Kindertransport program that rescued 10,000 children from the Nazi regime. His summer jobs included discovering golden deposits in Canada and helping Polaroid explain how its instant moving picture worked. Although he is now an emeritus professor, he is still actively working on projects involving global warming and renewable energy.
Computational Chemical science in Drug Design
While the descriptions of bonding described in this chapter involve many theoretical concepts, they also take many practical, existent-globe applications. For example, drug design is an important field that uses our understanding of chemical bonding to develop pharmaceuticals. This interdisciplinary surface area of study uses biological science (understanding diseases and how they operate) to identify specific targets, such as a binding site that is involved in a illness pathway. By modeling the structures of the bounden site and potential drugs, computational chemists can predict which structures can fit together and how finer they will demark (see Figure vii). Thousands of potential candidates tin be narrowed down to a few of the about promising candidates. These candidate molecules are then carefully tested to decide side effects, how effectively they tin can exist transported through the body, and other factors. Dozens of important new pharmaceuticals have been discovered with the aid of computational chemical science, and new research projects are underway.
Molecular Orbital Free energy Diagrams
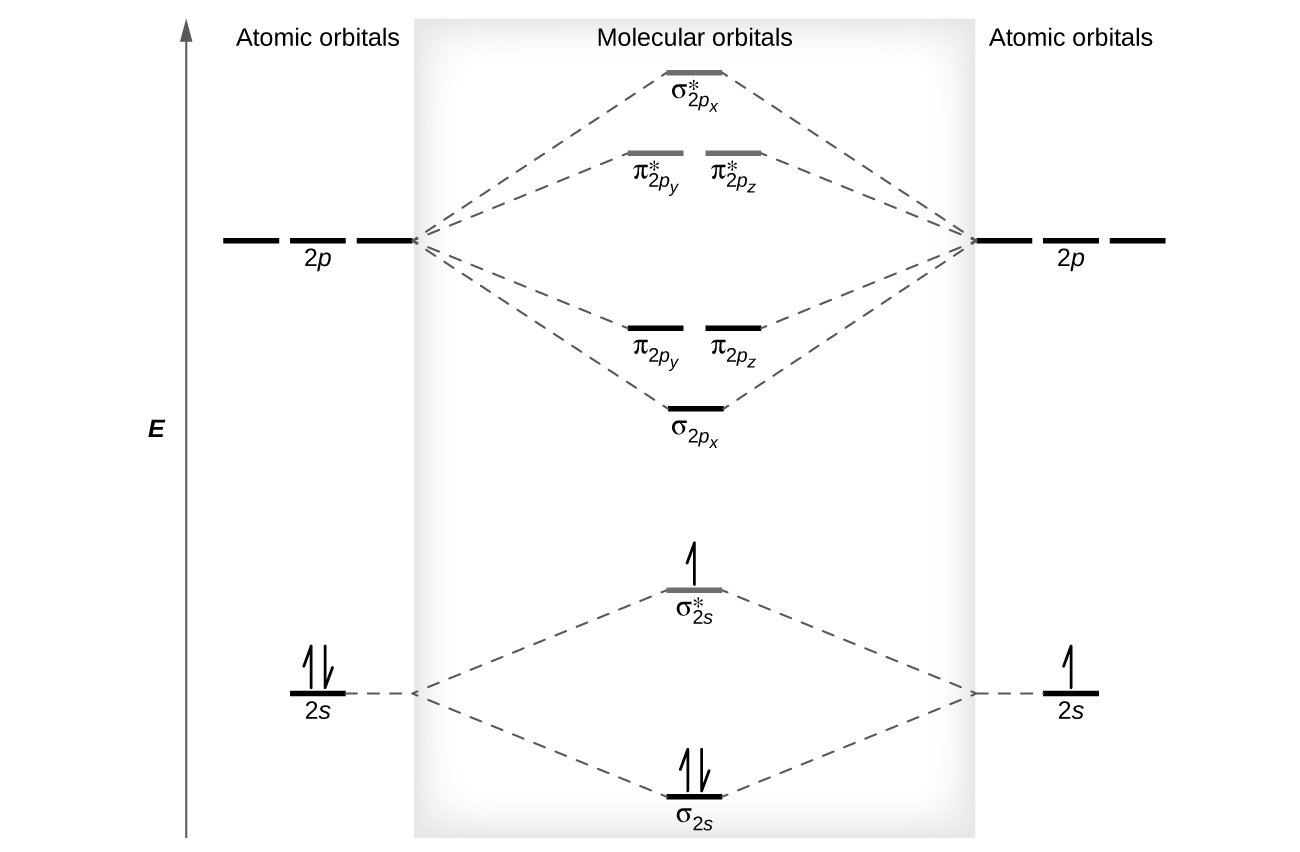
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Effigy 8). For a diatomic molecule, the diminutive orbitals of one atom are shown on the left, and those of the other cantlet are shown on the right. Each horizontal line represents one orbital that can agree two electrons. The molecular orbitals formed past the combination of the atomic orbitals are shown in the heart. Dashed lines prove which of the atomic orbitals combine to class the molecular orbitals. For each pair of atomic orbitals that combine, ane lower-free energy (bonding) molecular orbital and one college-energy (antibonding) orbital result. Thus we can see that combining the six 2p atomic orbitals results in iii bonding orbitals (ane σ and two π) and three antibonding orbitals (one σ* and two π*).
Nosotros predict the distribution of electrons in these molecular orbitals by filling the orbitals in the aforementioned way that nosotros fill atomic orbitals, by the Aufbau principle. Lower-energy orbitals fill first, electrons spread out among degenerate orbitals before pairing, and each orbital can agree a maximum of two electrons with contrary spins (Effigy viii). Merely as nosotros write electron configurations for atoms, we can write the molecular electronic configuration past listing the orbitals with superscripts indicating the number of electrons present. For clarity, we place parentheses around molecular orbitals with the same energy. In this case, each orbital is at a different free energy, and so parentheses separate each orbital. Thus we would await a diatomic molecule or ion containing vii electrons (such as Beii +) would take the molecular electron configuration [latex](\sigma_{1s})^two[/latex] [latex](\sigma^*_{1s})^2[/latex] [latex](\sigma_{2s})^two[/latex] [latex](\sigma^*_{2s})^i[/latex]. It is common to omit the cadre electrons from molecular orbital diagrams and configurations and include only the valence electrons.
Bond Order
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified past determining the bond order that results from the filling of the molecular orbitals by electrons.
When using Lewis structures to describe the distribution of electrons in molecules, we define bond order as the number of bonding pairs of electrons between two atoms. Thus a unmarried bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3. We define bail lodge differently when nosotros utilise the molecular orbital clarification of the distribution of electrons, but the resulting bond society is usually the same. The MO technique is more accurate and can handle cases when the Lewis structure method fails, simply both methods describe the same miracle.
In the molecular orbital model, an electron contributes to a bonding interaction if it occupies a bonding orbital and information technology contributes to an antibonding interaction if information technology occupies an antibonding orbital. The bond order is calculated by subtracting the destabilizing (antibonding) electrons from the stabilizing (bonding) electrons. Since a bond consists of two electrons, we divide by two to get the bail guild. Nosotros can determine bond order with the following equation:
[latex]\text{bail club} = \frac{(\text{number of bonding electrons}) - (\text{number of antibonding electrons})}{2}[/latex]
The order of a covalent bail is a guide to its strength; a bail between ii given atoms becomes stronger as the bail order increases (Table ane in Chapter 8.one Valence Bond Theory). If the distribution of electrons in the molecular orbitals between two atoms is such that the resulting bond would accept a bond society of zero, a stable bond does not form. We next await at some specific examples of MO diagrams and bond orders.
Bonding in Diatomic Molecules
A dihydrogen molecule (H2) forms from two hydrogen atoms. When the diminutive orbitals of the ii atoms combine, the electrons occupy the molecular orbital of lowest energy, the σ1s bonding orbital. A dihydrogen molecule, Hii, readily forms because the energy of a Hii molecule is lower than that of 2 H atoms. The σidue south orbital that contains both electrons is lower in energy than either of the two 1s atomic orbitals.
A molecular orbital tin can hold ii electrons, so both electrons in the Htwo molecule are in the σanes bonding orbital; the electron configuration is [latex](\sigma_{1s})^ii[/latex]. We stand for this configuration by a molecular orbital energy diagram (Figure 9) in which a single upwardly pointer indicates i electron in an orbital, and 2 (upward and downwardly) arrows point two electrons of opposite spin.
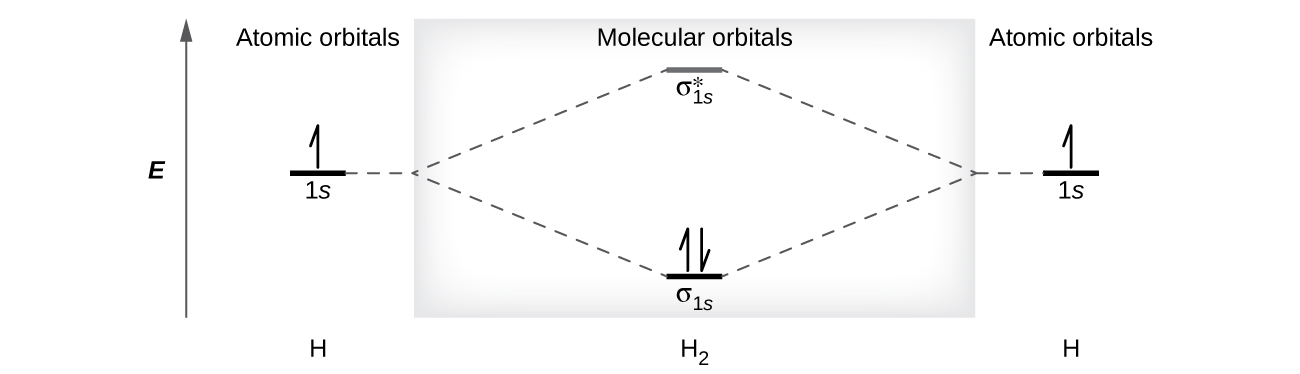
A dihydrogen molecule contains two bonding electrons and no antibonding electrons so nosotros have
[latex]\text{bond society in H}_2 = \frac{(ii - 0)}{two} = 1[/latex]
Because the bond guild for the H–H bond is equal to 1, the bond is a unmarried bond.
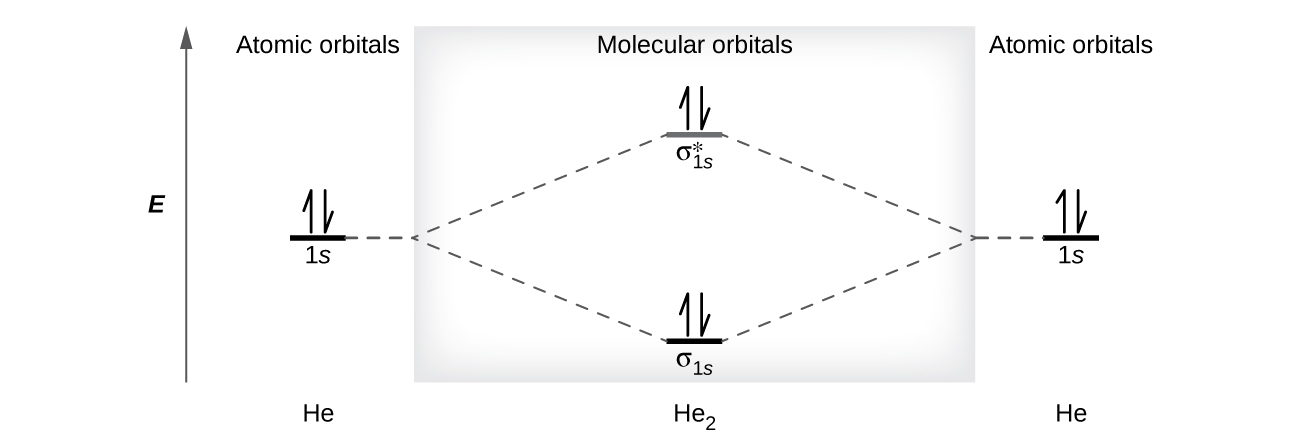
A helium atom has ii electrons, both of which are in its 1s orbital. Two helium atoms do not combine to form a dihelium molecule, He2, with four electrons, because the stabilizing event of the two electrons in the lower-energy bonding orbital would be offset by the destabilizing effect of the two electrons in the higher-energy antibonding molecular orbital. We would write the hypothetical electron configuration of He2 as [latex](\sigma_{1s})^2[/latex] [latex](\sigma^*_{1s})^two[/latex] as in Figure 10. The net free energy change would be zero, and so at that place is no driving forcefulness for helium atoms to form the diatomic molecule. In fact, helium exists as detached atoms rather than equally diatomic molecules. The bond order in a hypothetical dihelium molecule would be goose egg.
[latex]\text{bond order in He}_2 = \frac{(2 - two)}{2} = 0[/latex]
A bail order of cipher indicates that no bond is formed between two atoms.
The Diatomic Molecules of the Second Period
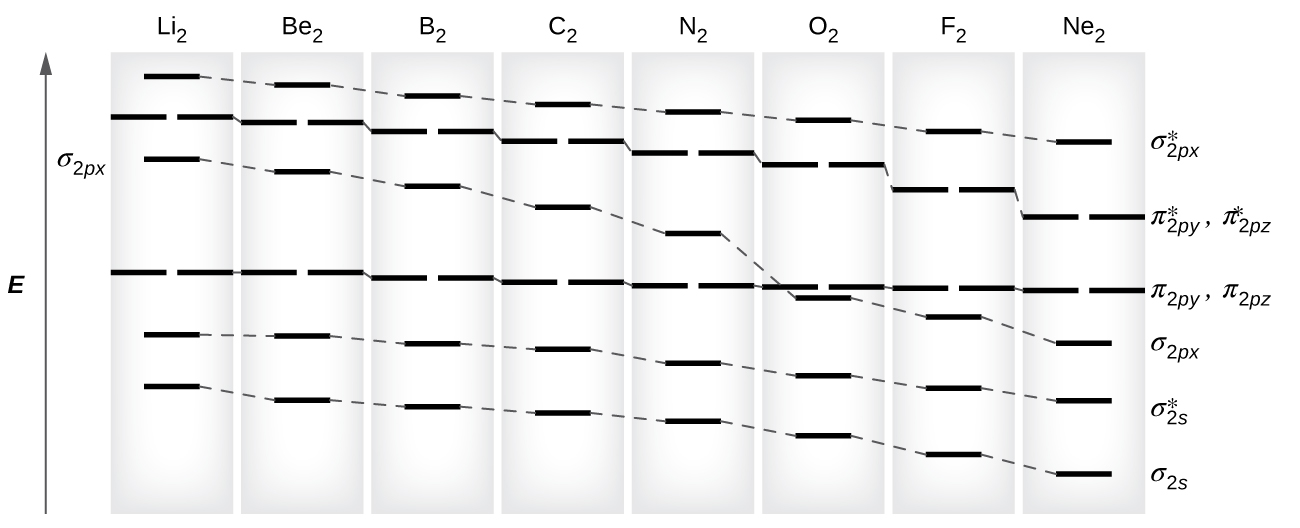
8 possible homonuclear diatomic molecules might be formed past the atoms of the second period of the periodic table: Li2, Beii, B2, Cii, Northwardii, O2, Ftwo, and Netwo. However, nosotros can predict that the Exist2 molecule and the Ne2 molecule would not exist stable. Nosotros can see this by a consideration of the molecular electron configurations (Tabular array 3).
We predict valence molecular orbital electron configurations simply every bit we predict electron configurations of atoms. Valence electrons are assigned to valence molecular orbitals with the lowest possible energies. Consistent with Hund'south rule, whenever there are 2 or more degenerate molecular orbitals, electrons fill each orbital of that type singly before any pairing of electrons takes place.
As nosotros saw in valence bail theory, σ bonds are mostly more stable than π bonds formed from degenerate atomic orbitals. Similarly, in molecular orbital theory, σ orbitals are usually more stable than π orbitals. All the same, this is not ever the case. The MOs for the valence orbitals of the second period are shown in Figure 11. Looking at Ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Still, for atoms with three or fewer electrons in the p orbitals (Li through N) we find a different pattern, in which the σ p orbital is higher in energy than the π p fix. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.

Y'all tin practice labeling and filling molecular orbitals with this interactive tutorial from the University of Sydney.
This switch in orbital ordering occurs considering of a phenomenon chosen s-p mixing. s-p mixing does non create new orbitals; information technology merely influences the energies of the existing molecular orbitals. The σs wavefunction mathematically combines with the σp wavefunction, with the outcome that the σs orbital becomes more stable, and the σp orbital becomes less stable (Effigy 12). Similarly, the antibonding orbitals besides undergo due south-p mixing, with the σdue south* becoming more than stable and the σp* becoming less stable.
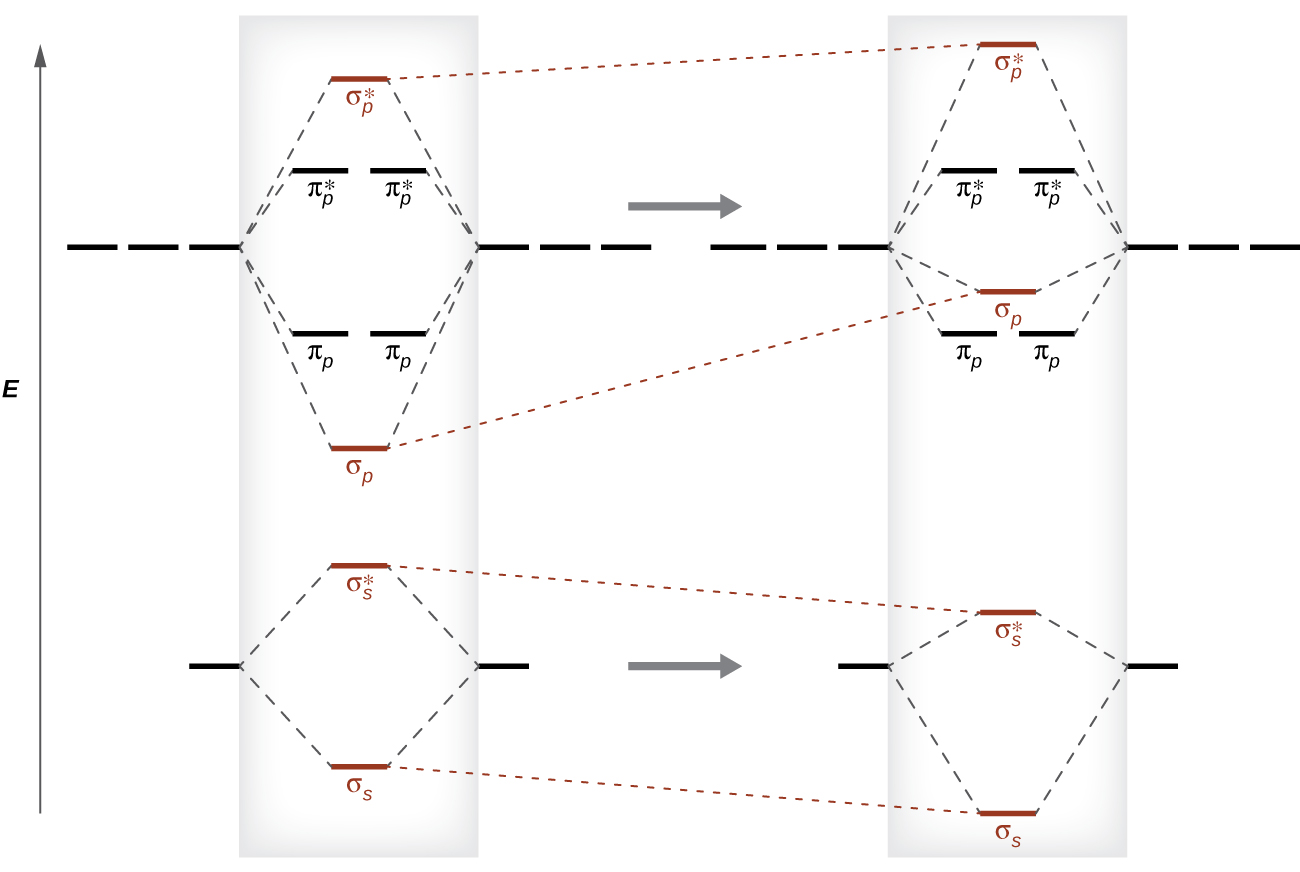
southward-p mixing occurs when the due south and p orbitals have like energies. When a unmarried p orbital contains a pair of electrons, the act of pairing the electrons raises the energy of the orbital. Thus the 2p orbitals for O, F, and Ne are higher in energy than the 2p orbitals for Li, Be, B, C, and N. Because of this, Otwo, F2, and N2 only take negligible s-p mixing (non sufficient to change the free energy ordering), and their MO diagrams follow the normal pattern, as shown in Figure eleven. All of the other period 2 diatomic molecules exercise accept s-p mixing, which leads to the pattern where the σp orbital is raised in a higher place the πp fix.
Using the MO diagrams shown in Effigy 11, we tin add in the electrons and determine the molecular electron configuration and bail order for each of the diatomic molecules. As shown in Table 3, Be2 and Ne2 molecules would have a bail order of 0, and these molecules exercise not exist.
| Molecule | Electron Configuration | Bond Club |
|---|---|---|
| Litwo | [latex](\sigma_{2s})^2[/latex] | 1 |
| Betwo (unstable) | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2[/latex] | 0 |
| B2 | [latex](\sigma_{2s})^ii (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^two[/latex] | 1 |
| Cii | [latex](\sigma_{2s})^two (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4[/latex] | 2 |
| Northward2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^two (\pi_{2py}, \pi_{2pz})^four (\sigma_{2px})^2[/latex] | iii |
| O2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2px})^ii (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^2[/latex] | ii |
| Fii | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2px})^2 (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^4[/latex] | i |
| Ne2 (unstable) | [latex](\sigma_{2s})^ii (\sigma^*_{2s})^2 (\sigma_{2px})^two (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^iv (\sigma^*_{2px})^2[/latex] | 0 |
| Table 3. Electron Configuration and Bond Order for Molecular Orbitals in Homonuclear Diatomic Molecules of Period Two Elements | ||
The combination of 2 lithium atoms to form a lithium molecule, Li2, is coordinating to the formation of Htwo, but the diminutive orbitals involved are the valence iisouthward orbitals. Each of the two lithium atoms has one valence electron. Hence, we have two valence electrons available for the σ2south bonding molecular orbital. Considering both valence electrons would be in a bonding orbital, nosotros would predict the Li2 molecule to be stable. The molecule is, in fact, present in appreciable concentration in lithium vapor at temperatures near the boiling point of the element. All of the other molecules in Table 3 with a bond order greater than zero are also known.
The Oii molecule has enough electrons to one-half fill up the ([latex]\pi^*_{2py}[/latex], [latex]\pi^*_{2pz}[/latex]) level. Nosotros expect the two electrons that occupy these two degenerate orbitals to be unpaired, and this molecular electronic configuration for O2 is in accord with the fact that the oxygen molecule has 2 unpaired electrons (Figure 14). The presence of two unpaired electrons has proved to exist difficult to explain using Lewis structures, but the molecular orbital theory explains it quite well. In fact, the unpaired electrons of the oxygen molecule provide a strong piece of support for the molecular orbital theory.
Band Theory
When two identical atomic orbitals on different atoms combine, two molecular orbitals upshot (encounter Figure iii). The bonding orbital is lower in energy than the original atomic orbitals considering the diminutive orbitals are in-phase in the molecular orbital. The antibonding orbital is higher in free energy than the original atomic orbitals considering the atomic orbitals are out-of-phase.
In a solid, similar things happen, just on a much larger scale. Remember that even in a modest sample at that place are a huge number of atoms (typically > x23 atoms), and therefore a huge number of atomic orbitals that may be combined into molecular orbitals. When Due north valence atomic orbitals, all of the same energy and each containing 1 (ane) electron, are combined, N/2 (filled) bonding orbitals and North/2 (empty) antibonding orbitals volition result. Each bonding orbital will show an energy lowering as the atomic orbitals are mostly in-stage, but each of the bonding orbitals will be a little different and have slightly dissimilar energies. The antibonding orbitals will evidence an increment in energy as the atomic orbitals are mostly out-of-phase, only each of the antibonding orbitals will too exist a niggling dissimilar and take slightly different energies. The allowed energy levels for all the bonding orbitals are so shut together that they course a ring, called the valence band. Likewise, all the antibonding orbitals are very shut together and form a band, called the conduction band. Figure thirteen shows the bands for 3 important classes of materials: insulators, semiconductors, and conductors.
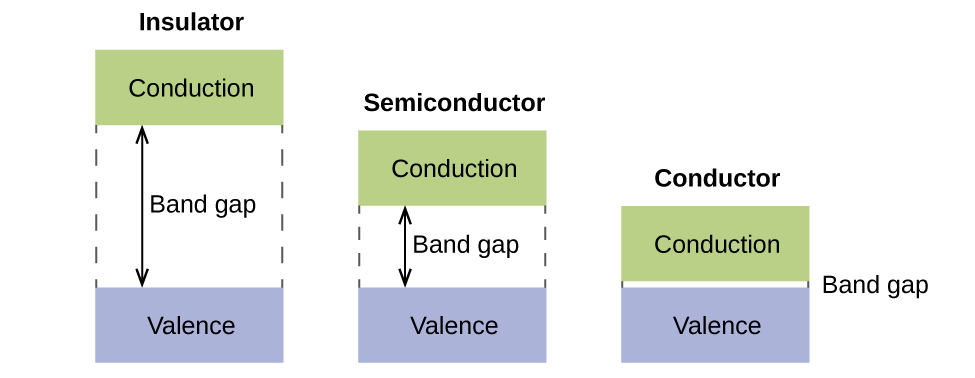
In lodge to comport electricity, electrons must move from the filled valence band to the empty conduction band where they tin can move throughout the solid. The size of the band gap, or the free energy difference between the top of the valence band and the bottom of the conduction band, determines how easy it is to move electrons betwixt the bands. Only a small-scale amount of energy is required in a conductor because the band gap is very small. This modest energy difference is "easy" to overcome, so they are good conductors of electricity. In an insulator, the band gap is so "large" that very few electrons motility into the conduction band; as a result, insulators are poor conductors of electricity. Semiconductors conduct electricity when "moderate" amounts of free energy are provided to move electrons out of the valence ring and into the conduction band. Semiconductors, such every bit silicon, are found in many electronics.
Semiconductors are used in devices such every bit computers, smartphones, and solar cells. Solar cells produce electricity when light provides the energy to move electrons out of the valence ring. The electricity that is generated may then exist used to power a lite or tool, or it can be stored for later use by charging a battery. As of December 2014, up to 46% of the energy in sunlight could be converted into electricity using solar cells.
Example 2
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons
Depict the molecular orbital diagram for the oxygen molecule, Otwo. From this diagram, calculate the bond order for Otwo. How does this diagram account for the paramagnetism of O2?
Solution
We draw a molecular orbital free energy diagram like to that shown in Figure xi. Each oxygen atom contributes 6 electrons, and then the diagram appears as shown in Effigy fourteen.
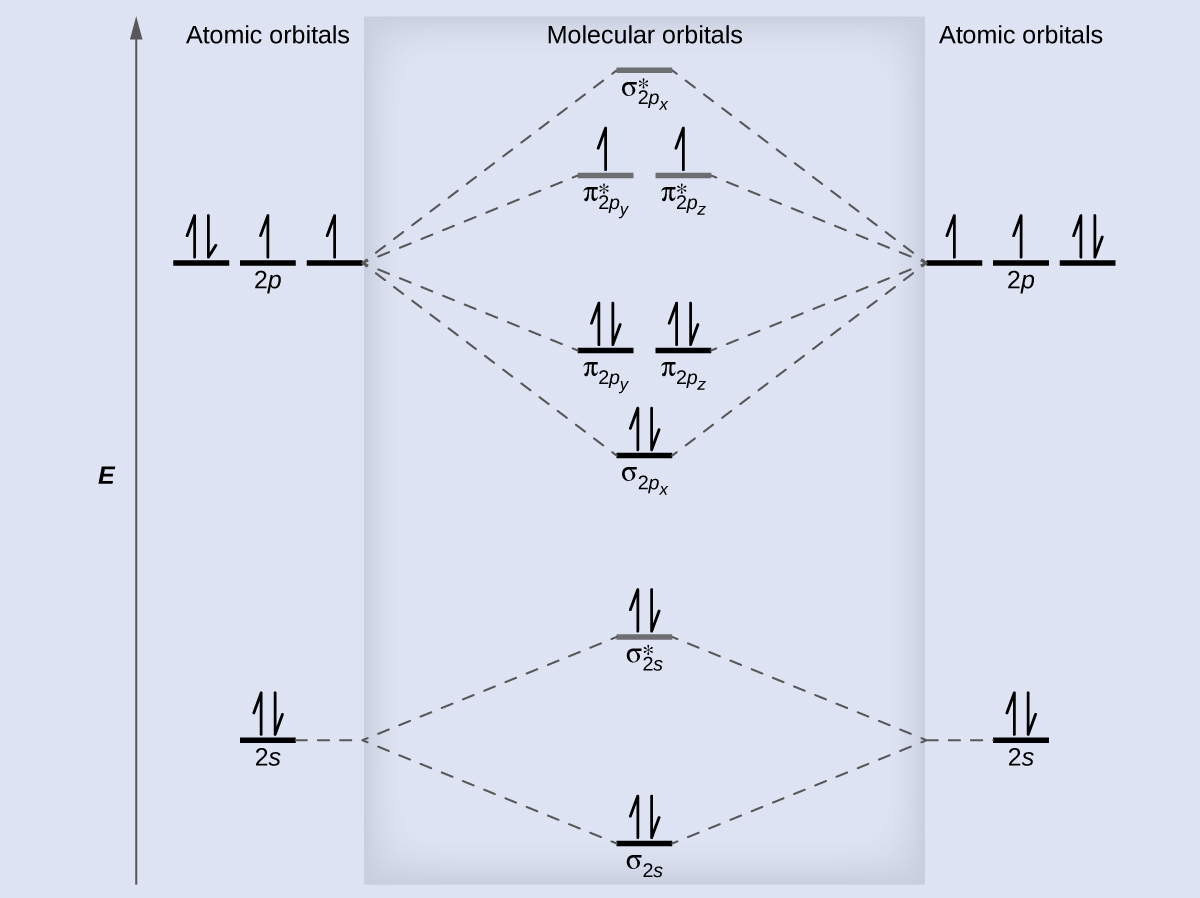
We summate the bond order as
[latex]\text{O}_2 = \frac{viii - 4}{two} = 2[/latex]
Oxygen'south paramagnetism is explained by the presence of two unpaired electrons in the (π2py , π2pz )* molecular orbitals.
Cheque Your Learning
The main component of air is Ntwo. From the molecular orbital diagram of N2, predict its bond order and whether information technology is diamagnetic or paramagnetic.
Reply:
Due north2 has a bond club of three and is diamagnetic.
Instance three
Ion Predictions with MO Diagrams
Give the molecular orbital configuration for the valence electrons in C2 2−. Will this ion be stable?
Solution
Looking at the appropriate MO diagram, we run into that the π orbitals are lower in energy than the σ p orbital. The valence electron configuration for C2 is [latex](\sigma_{2s})^2 (\sigma^*_{2s})^ii (\pi_{2py}, \pi_{2pz})^4[/latex]. Adding two more than electrons to generate the C2 ii− anion will requite a valence electron configuration of [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4 (\sigma_{2px})^ii[/latex]. Since this has six more bonding electrons than antibonding, the bond order will be 3, and the ion should be stable.
Bank check Your Learning
How many unpaired electrons would be present on a Exist2 2− ion? Would it be paramagnetic or diamagnetic?
Respond:
2, paramagnetic

Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas every bit the diatomic examples presented hither. However, with more atoms, computers are required to summate how the atomic orbitals combine. See iii-dimensional drawings of the molecular orbitals for C6H6.
Key Concepts and Summary
Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. The resulting molecular orbitals may extend over all the atoms in the molecule. Bonding molecular orbitals are formed by in-phase combinations of atomic moving ridge functions, and electrons in these orbitals stabilize a molecule. Antibonding molecular orbitals effect from out-of-stage combinations of diminutive wave functions and electrons in these orbitals make a molecule less stable. Molecular orbitals located along an internuclear axis are called σ MOs. They tin can be formed from s orbitals or from p orbitals oriented in an stop-to-terminate fashion. Molecular orbitals formed from p orbitals oriented in a side-by-side fashion have electron density on reverse sides of the internuclear axis and are called π orbitals.
We can describe the electronic construction of diatomic molecules by applying molecular orbital theory to the valence electrons of the atoms. Electrons fill molecular orbitals post-obit the same rules that apply to filling atomic orbitals; Hund's rule and the Aufbau principle tell us that lower-energy orbitals will fill up commencement, electrons volition spread out before they pair upwards, and each orbital tin agree a maximum of two electrons with opposite spins. Materials with unpaired electrons are paramagnetic and attracted to a magnetic field, while those with all-paired electrons are diamagnetic and repelled by a magnetic field. Correctly predicting the magnetic properties of molecules is in advantage of molecular orbital theory over Lewis structures and valence bond theory.
Cardinal Equations
- [latex]\text{bond society} = \frac{(\text{number of bonding electron}) - (\text{number of antibonding electrons})}{2}[/latex]
Chemistry End of Chapter Exercises
- Sketch the distribution of electron density in the bonding and antibonding molecular orbitals formed from two s orbitals and from two p orbitals.
- How are the following like, and how practice they differ?
(a) σ molecular orbitals and π molecular orbitals
(b) ψ for an diminutive orbital and ψ for a molecular orbital
(c) bonding orbitals and antibonding orbitals
- If molecular orbitals are created by combining five atomic orbitals from cantlet A and five atomic orbitals from atom B combine, how many molecular orbitals volition result?
- Tin can a molecule with an odd number of electrons ever be diamagnetic? Explain why or why not.
- Can a molecule with an even number of electrons e'er be paramagnetic? Explain why or why non.
- Why are bonding molecular orbitals lower in energy than the parent atomic orbitals?
- Calculate the bond order for an ion with this configuration:
[latex](\sigma_{2s})^ii (\sigma^*_{2s})^two (\sigma_{2px})^two (\pi_{2py} , \pi_{2pz})^four (\pi^*_{2py} , \pi^*_{2pz})^3[/latex]
- Explain why an electron in the bonding molecular orbital in the H2 molecule has a lower energy than an electron in the 1due south atomic orbital of either of the separated hydrogen atoms.
- Predict the valence electron molecular orbital configurations for the post-obit, and country whether they will be stable or unstable ions.
(a) Na2 2+
(b) Mg2 2+
(c) Al2 2+
(d) Si2 2+
(eastward) Pii 2+
(f) S2 2+
(thou) F2 2+
(h) Ar2 2+
- Determine the bond order of each fellow member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond.
(a) H2, H2 +, H2 −
(b) O2, Otwo 2+, O2 ii−
(c) Liii, Betwo +, Be2
(d) Ftwo, F2 +, F2 −
(e) N2, North2 +, Northward2 −
- For the commencement ionization energy for an Due northii molecule, what molecular orbital is the electron removed from?
- Compare the diminutive and molecular orbital diagrams to place the member of each of the following pairs that has the highest first ionization energy (the most tightly jump electron) in the gas phase:
(a) H and H2
(b) Northward and N2
(c) O and O2
(d) C and C2
(eastward) B and B2
- Which of the period 2 homonuclear diatomic molecules are predicted to be paramagnetic?
- A friend tells you that the iis orbital for fluorine starts off at a much lower free energy than the 2s orbital for lithium, and so the resulting σiisouth molecular orbital in F2 is more stable than in Li2. Do you agree?
- Truthful or false: Boron contains 2s 22p 1 valence electrons, so merely one p orbital is needed to form molecular orbitals.
- What charge would exist needed on F2 to generate an ion with a bail order of 2?
- Predict whether the MO diagram for Due south2 would show southward-p mixing or not.
- Explain why Nii 2+ is diamagnetic, while O2 four+, which has the same number of valence electrons, is paramagnetic.
- Using the MO diagrams, predict the bond order for the stronger bail in each pair:
(a) B2 or B2 +
(b) F2 or F2 +
(c) O2 or Otwo 2+
(d) Ctwo + or Cii −
Glossary
- antibonding orbital
- molecular orbital located outside of the region betwixt 2 nuclei; electrons in an antibonding orbital destabilize the molecule
- bond order
- number of pairs of electrons between two atoms; information technology tin exist establish by the number of bonds in a Lewis construction or by the departure between the number of bonding and antibonding electrons divided past 2
- bonding orbital
- molecular orbital located betwixt two nuclei; electrons in a bonding orbital stabilize a molecule
- degenerate orbitals
- orbitals that have the same free energy
- diamagnetism
- phenomenon in which a material is non magnetic itself simply is repelled past a magnetic field; it occurs when there are only paired electrons present
- homonuclear diatomic molecule
- molecule consisting of ii identical atoms
- linear combination of atomic orbitals
- technique for combining atomic orbitals to create molecular orbitals
- molecular orbital
- region of space in which an electron has a high probability of being plant in a molecule
- molecular orbital diagram
- visual representation of the relative energy levels of molecular orbitals
- molecular orbital theory
- model that describes the behavior of electrons delocalized throughout a molecule in terms of the combination of diminutive wave functions
- paramagnetism
- phenomenon in which a material is not magnetic itself merely is attracted to a magnetic field; it occurs when there are unpaired electrons present
- π bonding orbital
- molecular orbital formed by side-by-side overlap of atomic orbitals, in which the electron density is found on opposite sides of the internuclear axis
- π* bonding orbital
- antibonding molecular orbital formed by out of phase side-past-side overlap of atomic orbitals, in which the electron density is institute on both sides of the internuclear axis, and there is a node between the nuclei
- σ bonding orbital
- molecular orbital in which the electron density is institute along the axis of the bond
- σ* bonding orbital
- antibonding molecular orbital formed past out-of-phase overlap of diminutive orbital along the axis of the bond, generating a node between the nuclei
- s-p mixing
- change that causes σ p orbitals to exist less stable than π p orbitals due to the mixing of s and p-based molecular orbitals of similar energies.
Solutions
Answers to Chemical science Terminate of Chapter Exercises
2. (a) Similarities: Both are bonding orbitals that tin can comprise a maximum of 2 electrons. Differences: σ orbitals are terminate-to-end combinations of atomic orbitals, whereas π orbitals are formed past side-past-side overlap of orbitals. (b) Similarities: Both are quantum-mechanical constructs that stand for the probability of finding the electron about the cantlet or the molecule. Differences: ψ for an atomic orbital describes the beliefs of merely one electron at a time based on the atom. For a molecule, ψ represents a mathematical combination of atomic orbitals. (c) Similarities: Both are orbitals that can contain ii electrons. Differences: Bonding orbitals result in holding 2 or more atoms together. Antibonding orbitals have the upshot of destabilizing any bonding that has occurred.
4. An odd number of electrons can never be paired, regardless of the arrangement of the molecular orbitals. Information technology will ever exist paramagnetic.
6. Bonding orbitals have electron density in close proximity to more than than i nucleus. The interaction between the bonding positively charged nuclei and negatively charged electrons stabilizes the system.
eight. The pairing of the ii bonding electrons lowers the energy of the organisation relative to the energy of the nonbonded electrons.
10. (a) Htwo bond gild = one, Htwo + bond order = 0.5, Htwo − bond order = 0.five, strongest bond is Hii; (b) Oii bail guild = two, O2 2+ bond club = 3; O2 ii− bond order = 1, strongest bond is O2 2+; (c) Litwo bond order = one, Exist2 + bond order = 0.5, Beii bond club = 0, strongest bail is Li2;(d) F2 bail order = i, F2 + bond order = i.5, F2 − bond guild = 0.v, strongest bond is F2 +; (eastward) Northward2 bail gild = iii, Nii + bond order = 2.5, Due north2 − bond order = ii.5, strongest bail is Ntwo
12. (a) H2; (b) N2; (c) O; (d) C2; (e) Bii
14. Yep, fluorine is a smaller atom than Li, and then atoms in the 2s orbital are closer to the nucleus and more stable.
16. 2+
18. Due north2 has s-p mixing, then the π orbitals are the last filled in N2 ii+. O2 does not take south-p mixing, so the σ p orbital fills before the π orbitals.
Source: https://opentextbc.ca/chemistry/chapter/8-4-molecular-orbital-theory/
0 Response to "Use the Mo Diagram Provided Below to Answer the Following Questions: What Is the Bond Order for C2?"
Post a Comment